Tuesday, March 23, 2021
Starnberg, Germany, March 23rd, 2021 – PARI Pharma GmbH, a company focused on advanced aerosol delivery systems based on eFlow Technology, announces the authorization of the LAMIRA Nebulizer System for delivery of Insmed’s drug product ARIKAYCE (amikacin liposome inhalation suspension) in Japan. Insmed was granted approval for ARIKAYCE by Japan’s Ministry of Health, Labor and Welfare (MHLW) on March 23, 2021. The approval of ARIKAYCE in Japan follows earlier approvals in the United States and Europe. LAMIRA is the first drug-specific eFlow Technology nebulizer registered beyond Europe and North America.
The LAMIRA Nebulizer System is specifically optimized for ARIKAYCE and therefore the only device intended to administer it. Key features of LAMIRA include a customized medication reservoir to hold a full 8.4 ml dose, a specifically tailored aerosol head for the aerosolization of ARIKAYCE, and the valved aerosol chamber.
ARIKAYCE is the first inhaled liposomal dispersion approved by the MHLW and the first and only therapy in Japan indicated for the treatment of patients with nontuberculous mycobacterial (NTM) lung disease caused by Mycobacterium avium complex (MAC) who have not sufficiently responded to prior treatment with a multidrug regimen.
“LAMIRA gives us the ability to offer an eFlow Technology nebulizer to patients beyond Europe and North America. We are delighted that appropriate patients in Japan suffering from NTM lung disease now have access to a new treatment option,” said Dr. Martin Knoch, President at PARI Pharma.
Yuji Orihara, General Manager, Japan, for Insmed adds: “We are pleased that our valuable partnership with PARI has enabled us to bring to market the first approved drug, ARIKAYCE, to be used with the LAMIRA Nebulizer Device specifically for the treatment of patients in Japan with refractory MAC lung disease.”
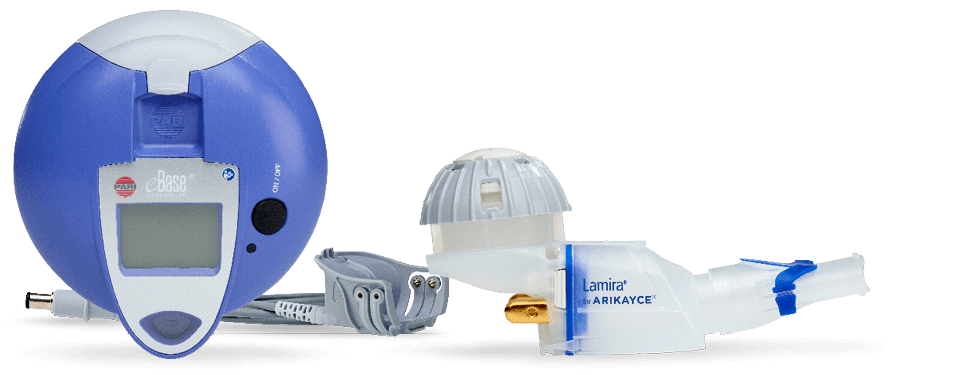
The LAMIRA® Nebulizer System, optimized for the administration of ARIKAYCE®, PARI Pharma GmbH
About PARI Pharma GmbH and eFlow Technology
PARI is a world leader in the development of aerosol delivery devices. PARI Pharma focuses on pharma licensing partnerships that utilize eFlow Technology nebulizers optimized for specific drug products and formulations.
eFlow Technology is an aerosol delivery platform that enables efficient nebulization of liquid medications via a vibrating, perforated membrane. eFlow Technology devices are designed to reduce the burden of treatment for patients with severe respiratory conditions.
About ARIKAYCE
ARIKAYCE is approved in the United States as ARIKAYCE® (amikacin liposome inhalation suspension), in the EU as ARIKAYCE® Liposomal 590 mg Nebuliser Dispersion, and in Japan as ARIKAYCE® Inhalation 590 mg (amikacin sulfate inhalation drug product). ARIKAYCE is a novel, inhaled, once-daily formulation of amikacin. Insmed's proprietary PULMOVANCE™ liposomal technology enables the delivery of amikacin directly to the lungs. ARIKAYCE is administered once daily using exclusively the LAMIRA Nebulizer System.

ARIKAYCE® is a registered trademark of Insmed Incorporated.
LAMIRA® is a registered trademark of PARI Pharma GmbH.
eFlow® is a registered trademark of PARI Pharma GmbH.
Contact information:
Michael Hahn
Vice President eFlow Partnering & Strategy
PARI Pharma GmbH
+49 (0) 89 74 28 46 -831
michael.hahnpari.com
www.pari.com/eflow-partnering
© 2024 PARI GmbH Spezialisten für effektive Inhalation