The use of CFTR modulators has transformed the treatment of cystic fibrosis in recent years. Patients who are eligible for triple CFTR modulator therapy perceive that their overall health is improving and stabilizing significantly.
In view of these advances, many people wonder whether their treatment – which for most of them has traditionally been inhalation therapy, physiotherapy and systemic drug therapy – can be adapted.
We spoke exclusively with CF experts Prof. Pierre-Régis Burgel from France and Prof. Francesco Blasi from Italy to find out their views.
Find out in the video interviews with Prof. Burgel and Prof. Blasi why antibiotic inhalation under the CFTR modulator triple combination is still important.
Prof. Pierre-Régis Burgel
is a renowned expert in respiratory medicine at the Institut Cochin, Université Paris Cité. He is involved in many clinical and basic research studies in CF-related issues.
Prof. Francesco Blasi
is a Professor of Respiratory Medicine in the Department of Pathophysiology and Transplantation at the University of Milan with acclaimed expertise in the lung microbiome. He was president of the European Respiratory Society and of the Italian Respiratory Society. Currently he serves as president of the Italian Cystic Fibrosis Society (SIFC).
In the current publication of the series "Standards for the care of CF patients", the European CF Society makes the following statement based on a Delphi survey [1]:
"There is good evidence to support eradication protocols for Pseudomonas aeruginosa identified on respiratory culture of any airway sample Significantly more eradications are achieved with nebulized antibiotics (± oral antibiotics) than without therapy. Intravenous antibiotics confer no advantage."
A renowned team of cystic fibrosis experts collaborating with J. Stuart Elborn (Faculty of Medicine Health and Life Sciences, Queen’s University, Belfast, UK) have now tackled this question. In their paper recently published in the European Respiratory Review, they address inhaled antibiotics in the era of CFTR modulators [2].
Their clear conclusion: Inhaled antibiotics are still recommended for patients receiving CFTR modulators who present with chronic infections. This is because there are no long-term data to prove that it is safe to stop taking antibiotics.
Role of inhaled antibiotics in the era of highly effective CFTR modulators
J. Stuart Elborn et al. European Respiratory Review 2023
382 KB Download PDFRecurrent or chronic bacterial infection with Pseudomonas aeruginosa harbours a host of risks. Bacterial airway infections are strongly associated with exacerbations, limited quality of life and increased mortality [3,4].
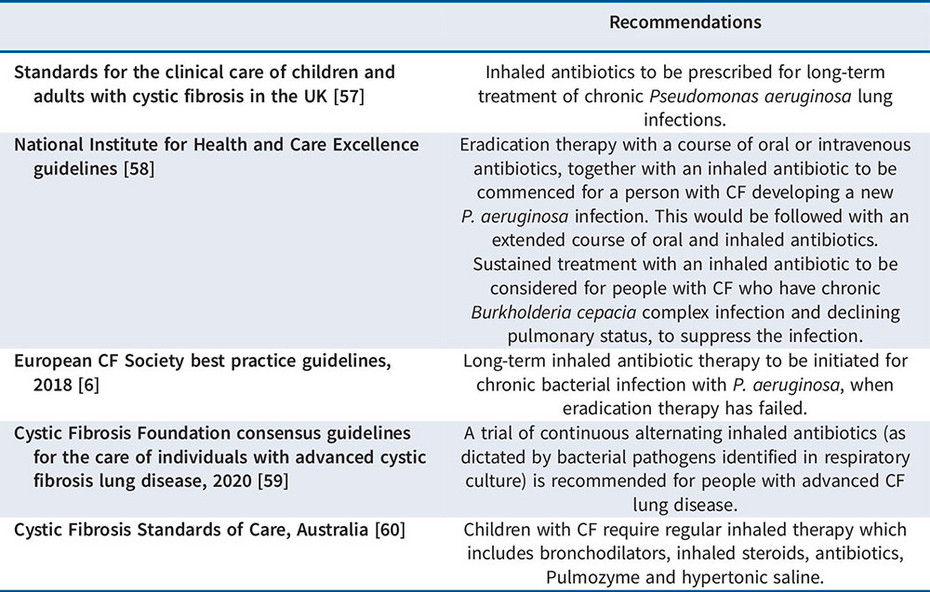
The use of inhaled antibiotics for cystic fibrosis patients is an evidence-based treatment with a long-standing track record in the management of chronic infection with Pseudomonas aeruginosa [5,6].
Early intervention with inhaled antibiotics, as monotherapy or combined with oral antibiotics, is also an effective strategy to delay chronic colonisation with Pseudomonas aeruginosa [5,6].
References
1 Burgel P-R et al.,J Cyst Fibros, epub 16 Jan 2024.
2 Elborn JS, Blasi F, Burgel P-R et al. Eur Respir Rev 2023; 32: 220154.
3 VanDevanter DR et al. J Cyst Fibros 2015; 14: 763–769.
4 Turcios NL Respir Care 2020; 65: 233–251.
5 Taccetti G et al. Antibiotics 2021; 10: 338.
6 Castellani C et al. J Cyst Fibros 2018; 17: 153–178.
© 2025 PARI GmbH Spezialisten für effektive Inhalation