Nebuliser therapy plays an important role in the therapy of different diseases of the airways such as cystic fibrosis or COPD. Its use is furthermore advantageous in particular for small children and older patients. Even during the COVID-19 pandemic, nebuliser therapy is recommended in national and international guidelines for the treatment of chronic airway disease.1–4 In hospitals this is increasingly accompanied with the use of filter systems to avoid possible aerosol emissions into the environment.4–6 Due to its scientifically proven and excellent filter characteristics,7 the PARI Filter Valve Set is a simple way to help minimise the potential risk for medical personnel working in hospitals.
Numerous patients with, e.g. cystic fibrosis or severe COPD are dependent on nebuliser therapy. Also patients with cognitive or physical limitations benefit particularly as they are often unable to correctly perform other inhalable measures such as using a pMDI or DPI.8,9
Various national guidelines, such as those of the British National Institute for Health and Care Excellence (NICE) recommend the continuation of nebuliser therapy during the COVID-19 pandemic.1–3 The COVID-19-Positioning Paper of the DPG (German society for pulmonology and respiratory medicine) points out that jet nebulisers in no way increase the risk of infection for medical personnel working in hospitals.10 Even the CDC (Centers for Disease Control and Prevention) questions any connection between nebuliser therapy and an increased risk of a COVID-19 infection for medical personnel.11 International experts have reached a similar conclusion whilst emphasising at the same time that nebulising isotonic saline can reduce the release of bioaerosols from the lung by an average of 72%. 4,10,12 In the meantime it is widely accepted that bioaerosols released by patients play an important role in the transmission of the virus.
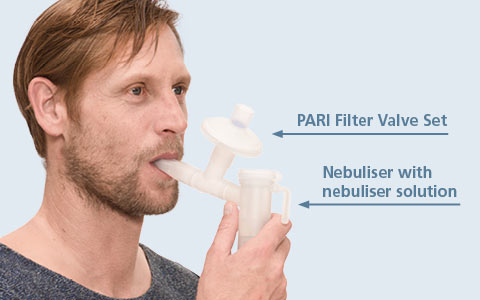
As hospitals in general are taking more precautionary measures to prevent the spread of infection, several national guidelines recommend 4–6,13 the use of an expiratory filter such as the PARI Filter Valve Set in nebuliser therapy. (See picture 1)
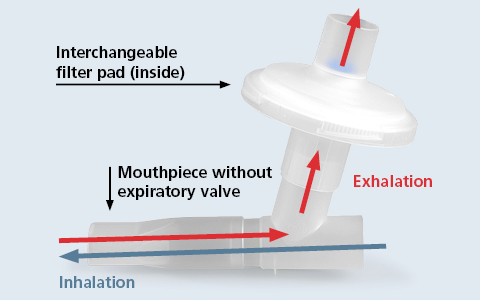
If using the PARI Filter Valve Set during nebuliser therapy, the medical aerosol is inhaled through the mouthpiece without valve (See picture 2: blue arrow). The air being exhaled is filtered through the pad (See picture 2: red arrow) Even very small aerosol particles can be captured. The retention rate of the PARI pads is equivalent to that of a FFP2 mask*.
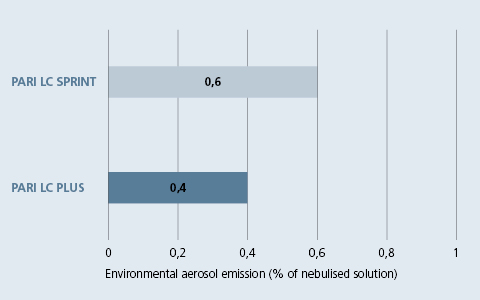
The efficiency of the PARI Filter Valve Set in combination with a PARI LC SPRINT or PARI LC PLUS nebuliser in reducing aerosol being released into the environment was tested in a current in-vitro study. The emitted aerosol was collected in an environmental filter located above the closed nebuliser as well as in the expiratory filter of the PARI Filter Valve Set.
It could be shown that the nebuliser with PARI Filter Valve Set released less than 1% of the nebulised solution into the environment. The PARI LC PLUS and PARI LC SPRINT nebulisers emitted on average 0.4% and 0.6% respectively. (See picture 3).7
The highly efficient filter is able to minimise the potential risk of aerosol released into the environment.
Use of the PARI Filter Valve Set can as such make a meaningful contribution to increase the safety of nebuliser therapy in hospitals. It can be used with the PARI LC SPRINT, PARI LC PLUS and the eFlow rapid nebuliser handset.
* Measured by manufacturer according to EN 13274-7with a flow velocity of 9,5m/min and NaCl aerosol particles with a median diameter of between 0,06 μm und 0,10 μm. Geometric standard deviation of between 2,0 und 3,0.
1. NICE National Institute for Health and Care Excellence. COVID-19 rapid guideline: community-based care of patients with chronic obstructive pulmonary disease (COPD). Published online April 9, 2020:13.
2. NICE National Institute for Health and Care Excellence. COVID-19 rapid guideline: cystic fibrosis. Cyst Fibros.:13.
3. NICE National Institute for Health and Care Excellence. COVID-19 rapid guideline: severe asthma. Sev Asthma.:12.
4. Fink JB, Ehrmann S, Li J, et al. Reducing Aerosol-Related Risk of Transmission in the Era of COVID-19: An Interim Guidance Endorsed by the International Society of Aerosols in Medicine. J Aerosol Med Pulm Drug Deliv. Published online August 12, 2020:jamp.2020.1615.
5. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting. Recommendations to guide clinical practice. J Physiother. 2020;(1).
6. Vitacca M, Lazzeri M, Guffanti E, et al. Italian suggestions for pulmonary rehabilitation in COVID-19 patients recovering from acute respiratory failure: results of a Delphi process. Monaldi Arch Chest Dis. 2020;90(2).
7. Schuschnig U, Ledermueller R, Gramann J. Efficacy of the PARI Filter Valve Set to Prevent Environmental Contamination with Aerosol during Nebulizer Therapy.; 2020.
8. Dhand R, Dolovich M, Chipps B, R. Myers T, Restrepo R, Rosen Farrar J. The Role of Nebulized Therapy in the Management of COPD: Evidence and Recommendations. COPD J Chronic Obstr Pulm Dis. 2012;9(1):58-72.
9. Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308-1417.
10. Pfeifer M, Ewig S, Voshaar T, et al. Position Paper for the State-of-the-Art Application of Respiratory Support in Patients with COVID-19. Respiration. 2020;99(6):521-542.
11. CDC (Centers for Disease Control and Prevention). Coronavirus disease 2019 (COVID-19) Healthcare infection prevention and control FAQs for COVID-19. www.cdc.gov/coronavirus/2019-ncov/ hcp/infection-control-faq.html.
12. Edwards DA, Man JC, Brand P, et al. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci. 2004;101(50):17383-17388.
13. Reychler G, Vecellio L, Dubus JC. Nebulization: A potential source of SARS-CoV-2 transmission. Respir Med Res. 2020;78:100778.
© 2025 PARI GmbH Spezialisten für effektive Inhalation