
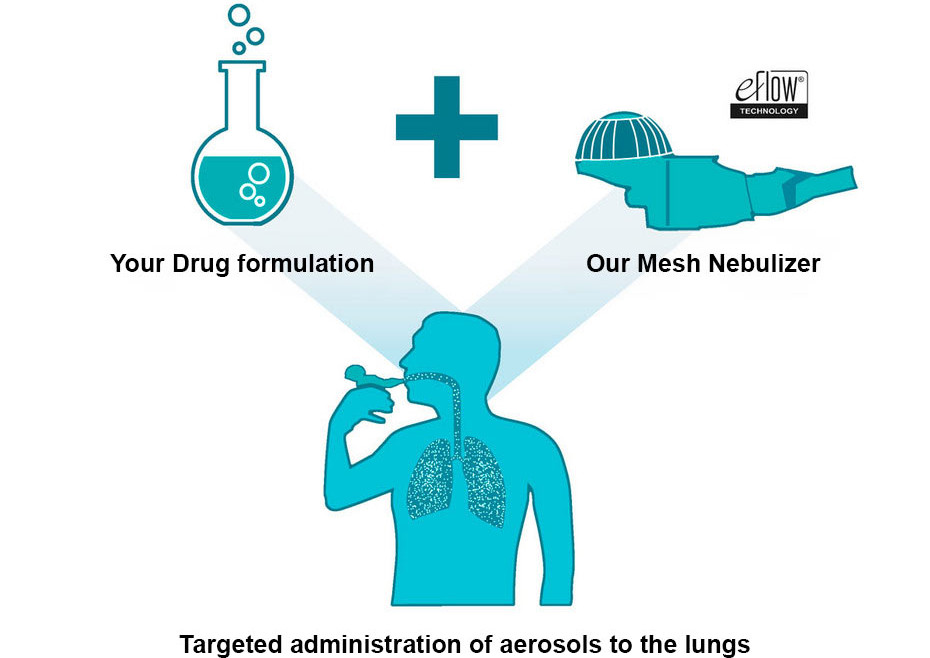
Bringing new innovative inhalation therapies to market is a long and risky process. The interaction of drug and device involves a high degree of complexity where both technical and regulatory requirements must be met. Having this in mind, choosing the ideal nebulizer and device partner can make all the difference and reduce overall development risks.
Rely on PARI as a trusted partner for the most suitable nebulizer - ensuring the best fit to your drug.
Investor (during ATS 2024 podium discussion)
CEO (during ERS 2024)
Learn what made Avalyn Pharma decide to go with a customized eFlow Technology nebulizer. Spoiler: ~8.5 min per dose with an eFlow vs ~27 DPI inhalations or 45–60 min on general-purpose nebulizers.

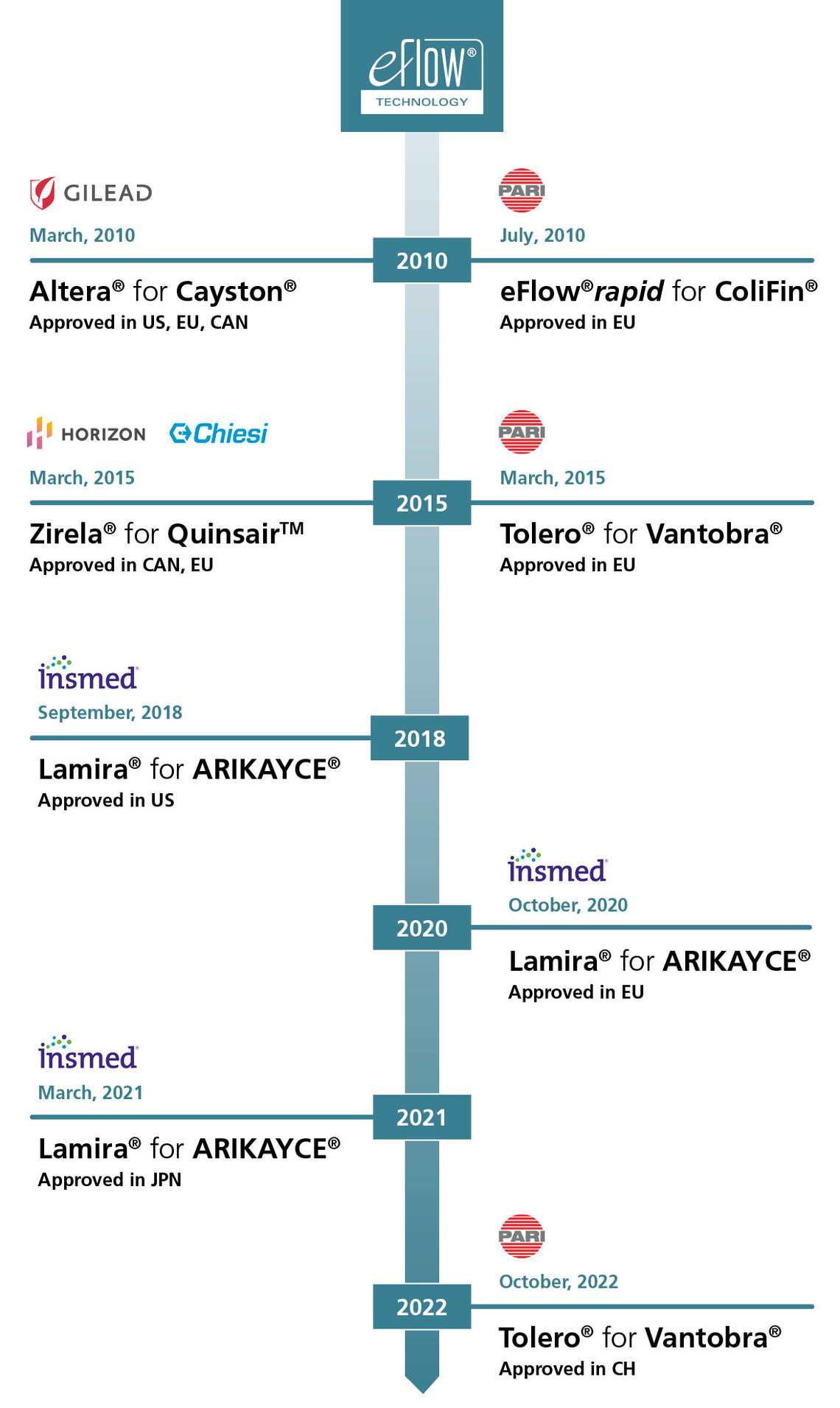
ARIKAYCE®*
Mycobacterium avium complex (MAC) Lung disease

L-CsA-I
Bronchiolitis Obliterans Syndrome (BOS) in Lung Transplantation

Inhaled CMS
Non-CF Bronchiectasis

Molgramostim
Autoimmune Pulmonary Alveolar Proteinosis (aPAP)

ARINA-1
Bronchiolitis Obliterans Syndrome (BOS) in Lung Transplantation

ARINA-1
Non-CF Bronchiectasis

AP01
Progressive Pulmonary Fibrosis (PPF)

RCT1100
Primary Ciliary Dyskinesia (PCD)



Discover how PARI supports you in achieving impactful clinical results and commercial success