
Ensuring the best Fusion of Your Formulation with our eFlow® Technology.
We use our extensive customization options to define the ideal nebulizer for your formulation. This reduces the overall risk and saves you time on the way to a commercial product.
Get more details from our experts on the evaluation phase and nebulizer customization:
Jonas Knoch, Business Development Manager eFlow, provides valuable insights into the early evaluation of drug formulation and nebulizer.
Dr. Markus Tservistas, Senior Project Leader eFlow, explains how the previous evaluation work is continued in the development stage and how we can support clinical trials and regulatory submissions.
Dr. Benjamin Heine, Technology Platform Leader eFlow, gives you an idea of the methods used to customize eFlow® Technology nebulizers for your drug formulation.

Highly efficient nebulizer platform, well-established in several commercial products.
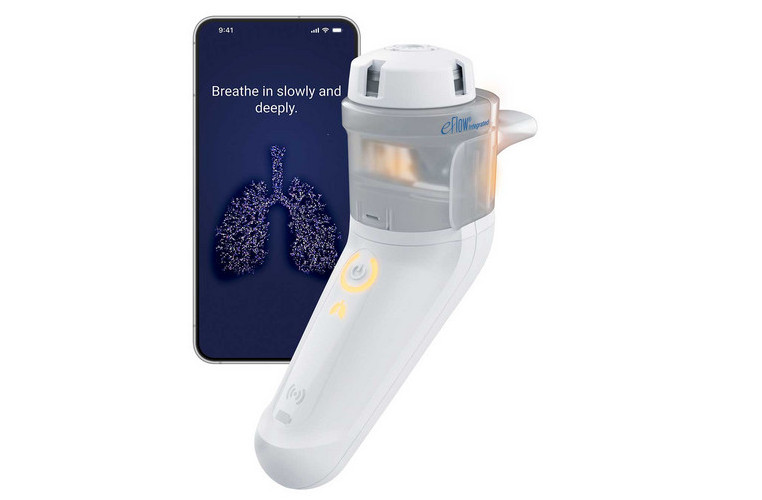
Innovative breath-triggered nebulizer platform with breath-guiding (under development).
Our eFlow Technology, refined over 20 years, features a laser-drilled vibrating mesh. Today the platform offers a wide range of validated meshes to optimize aerosol performance and ensure ideal delivery efficiency.
By tailoring aerosol parameters to your specific needs we can improve efficacy of your formulation.

